 sales: gm@sspseals.com Toll Free: +1-888-238-SEAL Request A Quote
sales: gm@sspseals.com Toll Free: +1-888-238-SEAL Request A Quote
Tri-Clamp® Mini is one of the many types of gaskets used in industries where sanitation is a major requirement. SSP Manufacturing, Inc. is a leading provider of these gaskets, which are made from materials that meet FDA approval.

We provide Tri-Clamp® Mini sanitary gaskets in following material options:
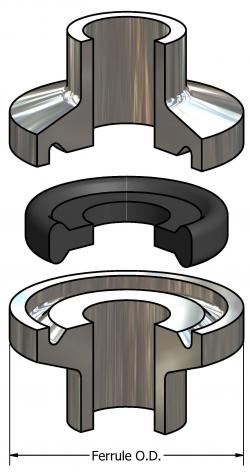
Tri-Clamp® Mini sanitary gaskets are used in various industries like:
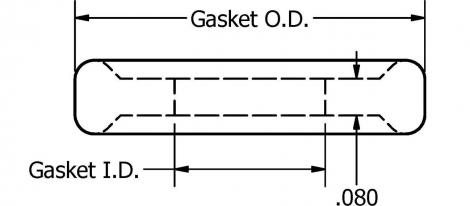
In sanitary process industry, Tri-clamp ® Mini is mainly used for sealing clamp connections in sanitary pipe lines. The dimensions of these types of gaskets are dependent on the application for which they are used.
At SSP Manufacturing, Inc. we understand the value of providing high-quality products, along with the best-in-class service. Valuable feedback from our stakeholders, and amazing response from our customers have helped us attain a distinguishable recognition in the industry. We are known to provide the best-quality tri-clamp ® Mini sanitary gaskets, and we never compromise on the quality. Please contact us by phone 888-238-SEAL or email at gm@sspseals.com, and our representative will address all your requests and queries.
SSP Manufacturing Inc ships all products with a certificate of conformance.
It is SSP Manufacturing Inc policy to provide the highest quality products, which consistently meet the product specifications developed by SSP Manufacturing Inc and their customers, both internal and external. We are committed to the continuous improvement of our quality system. We will meet and exceed the expectations of our customers.
Gaskets Meet the Most Stringent Standards For Purity:
*Buna does not pass U.S. Pharmacopeia Class VI Certification And Cytotoxicity and is not ADI Free®.